
Chemistry, 06.08.2019 02:30 jayjay9434
The equilibrium constant kp for the reaction pcl5(g) ⇌ pcl3(g) + cl2(g) is 1.05 atm at 250ºc. the reaction starts with a mixture of pcl5, pcl3, and cl2 at pressures 0.177 atm, 0.223 atm, and 0.111 atm, respectively, at 250ºc. when the mixture comes to equilibrium at that temperature, which pressures will have decreased and which will have increased? explain why.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 16:00
Is a measure of the resistance to flow. a high liquid has a high resistance to flow and flows slowly. the ancients thought everything in the world was made of 4 we now know that there are 94 naturally occurring and scientists have created another 24 i am certain they will create even more. honey flows slowly because it has a high to flowing. a can be separated by physical means because it contains more than one pure substance and 2 pure substances are not chemically bonded to each other. a cannot be separated by physical means. all matter is made up of all elements are with the same number of protons. if it is just a single or many bonded together, if all of them have the same number of protons, it is an element. in a piece of pure iron metal, all the are joined together, that piece of iron metal is called elemental iron. a single of iron is called elemental iron. a mixture has differences from place to place. we might need a microscope to see them or they might be obvious to the unaided eye. there are surfaces separating it into different phases. a mixture is the same everywhere. it is uniform. there are no surfaces separating it into different phases. if different kinds of atoms (different elements) are bonded together by their electrons, it is called a there are physical means of to isolate the different pure substances in a mixture and there are chemical means of to isolate the different elements in a compound. 1. element 2. compound 3. mixture 4. heterogeneous 5. homogeneous 6. pure substance 7. atoms 8. separation 9. viscosity 10. resistance
Answers: 2
You know the right answer?
The equilibrium constant kp for the reaction pcl5(g) ⇌ pcl3(g) + cl2(g) is 1.05 atm at 250ºc. the re...
Questions

Biology, 25.07.2019 23:50

History, 25.07.2019 23:50

Biology, 25.07.2019 23:50

Chemistry, 25.07.2019 23:50

Chemistry, 25.07.2019 23:50

English, 25.07.2019 23:50





Spanish, 25.07.2019 23:50

Mathematics, 25.07.2019 23:50


Health, 25.07.2019 23:50

Chemistry, 25.07.2019 23:50



English, 25.07.2019 23:50


History, 25.07.2019 23:50

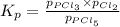
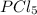 ,
, 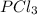 , and
, and 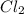 at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.
at pressures as 0.177 atm, 0.223 atm, and 0.111 atm, respectively.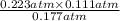
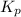 > 0.14 atm. As calculated value is less than the given value of
> 0.14 atm. As calculated value is less than the given value of 

