
Chemistry, 02.08.2019 20:20 dannyo6680
Suppose you have been given the task of distilling a mixture of hexane + toluene. pure hexane has a refractive index of 1.375 and pure toluene has a refactive index of 1.497. you collect a distillate sample which has a refractive index of 1.401. assuming that the refractive index of the hexane + toluene mixture varies linearly with mole fraction, what is the mole fraction of hexane in your sample?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
Suppose you have been given the task of distilling a mixture of hexane + toluene. pure hexane has a...
Questions





Physics, 01.12.2020 01:00


Mathematics, 01.12.2020 01:00



Chemistry, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00



English, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00





Mathematics, 01.12.2020 01:00

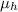 = 1.375
= 1.375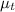 = 1.497
= 1.497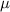 = 1.401
= 1.401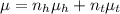 (1)
(1) = hexane mole fraction
= hexane mole fraction = toulene mole fraction
= toulene mole fraction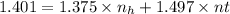 (3)
(3)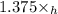 + 1.375\times
+ 1.375\times 

