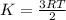Chemistry, 01.08.2019 18:10 reeseleprell7655
What is true of a sample of gas as temperature is increased?
the particles move faster, and their average kinetic energy decreases.
the particles move faster, and their average kinetic energy increases.
the particles move slower, and their average kinetic energy decreases.
the particles move slower, and their average kinetic energy increases.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:40
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2


Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
What is true of a sample of gas as temperature is increased?
the particles move faster, and t...
the particles move faster, and t...
Questions




Mathematics, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00







Chemistry, 29.10.2020 05:00


Mathematics, 29.10.2020 05:00

Mathematics, 29.10.2020 05:00



Chemistry, 29.10.2020 05:00