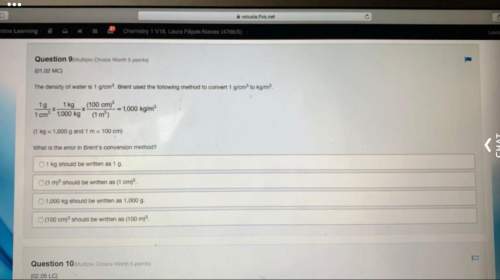
Need this asap!
the boiling point of water (h2o) is 100 °c. which of the following best predicts the boiling point of hydrogen sulfide (h2s)?
a. higher than 100 °c because ion dipole interactions in hydrogen sulfide are stronger than hydrogen bonding in water.
b. lower than 100 °c because hydrogen sulfide has dipole-dipole interactions instead of hydrogen bonding.
c. lower than 100 °c because the hydrogen bonding in hydrogen sulfide is weaker than it is in water.
d. higher than 100 °c because the size of a sulfur atom is larger than the size of an oxygen atom.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
You know the right answer?
Need this asap!
the boiling point of water (h2o) is 100 °c. which of the following best predi...
the boiling point of water (h2o) is 100 °c. which of the following best predi...
Questions


Mathematics, 29.09.2021 21:30



Chemistry, 29.09.2021 21:30


Mathematics, 29.09.2021 21:30



Chemistry, 29.09.2021 21:30

Mathematics, 29.09.2021 21:30



Mathematics, 29.09.2021 21:30


Biology, 29.09.2021 21:30


Mathematics, 29.09.2021 21:30

Mathematics, 29.09.2021 21:30