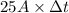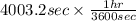Chemistry, 31.07.2019 18:20 chanel2371
Chromium plating can be applied by electrolysis to objects according to the following unbalanced half-reaction: cr2o72- + e- + h+→ cr(s) + h2ohow long (in hours) would it take to apply a chromium plating 0.010 mm thick to a car bumper with a surface area of 0.25 m2 in a cell with a current of 25.0 a? the density of chromium is 7.19 g/cm3.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
Chromium plating can be applied by electrolysis to objects according to the following unbalanced hal...
Questions




History, 23.09.2019 21:40

Biology, 23.09.2019 21:40

History, 23.09.2019 21:40

History, 23.09.2019 21:40








History, 23.09.2019 21:50

Mathematics, 23.09.2019 21:50



Mathematics, 23.09.2019 21:50

Computers and Technology, 23.09.2019 21:50

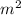 , current (I) = 25 A
, current (I) = 25 A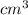
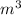 =
= 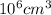
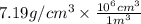
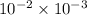 =
= 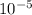 m.
m.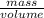
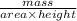
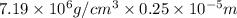
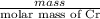
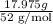
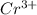 is 579000 C
is 579000 C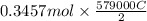 = 100080 C.
= 100080 C. 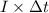
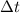 = time in seconds
= time in seconds