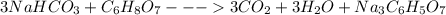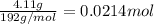Antacids, such as alka-seltzer, use the reaction of sodium bicarbonate with citric acid in water solution to produce a fizz as follows: 3nahco3 + c6h8o7 → 3co2 + 3h2o + na3c6h5o7 if 4.11 g of the citric acid (c6h8o7, mw = 192 g/mol) react with excess sodium bicarbonate (nahco3), how many grams of carbon dioxide (co2, mw = 44 g/mol) are formed as the solution fizzes?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
You know the right answer?
Antacids, such as alka-seltzer, use the reaction of sodium bicarbonate with citric acid in water sol...
Questions

Social Studies, 25.03.2020 18:59




Mathematics, 25.03.2020 18:59

Mathematics, 25.03.2020 18:59



History, 25.03.2020 18:59

Health, 25.03.2020 18:59


Biology, 25.03.2020 18:59

Engineering, 25.03.2020 18:59

English, 25.03.2020 18:59


English, 25.03.2020 18:59

Mathematics, 25.03.2020 18:59


Mathematics, 25.03.2020 18:59