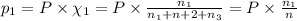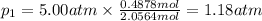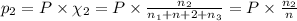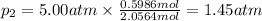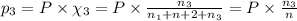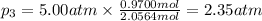Chemistry, 08.07.2019 21:10 cschellfamily
Three gases (8.00 g of methane, ch4, 18.0 g of ethane, c2h6, and an unknown amount of propane, c3h8) were added to the same 10.0-l container. at 23.0 ∘c, the total pressure in the container is 5.00 atm . calculate the partial pressure of each gas in the container.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1
You know the right answer?
Three gases (8.00 g of methane, ch4, 18.0 g of ethane, c2h6, and an unknown amount of propane, c3h8)...
Questions


Mathematics, 11.01.2021 06:40


Mathematics, 11.01.2021 06:40

English, 11.01.2021 06:40

Mathematics, 11.01.2021 06:40


Physics, 11.01.2021 06:40

English, 11.01.2021 06:40








Mathematics, 11.01.2021 06:40


Mathematics, 11.01.2021 06:40

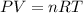 (Ideal gas equation)
(Ideal gas equation)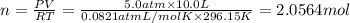
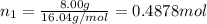
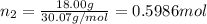
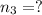
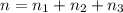
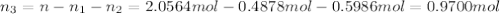
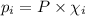
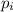 = partial pressure of 'i' component.
= partial pressure of 'i' component.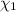 = mole fraction of 'i' component in mixture
= mole fraction of 'i' component in mixture