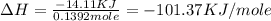Chemistry, 06.07.2019 19:10 angelZ3947
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g.
a. -101.37 kj
b. -7.05 kj
c. 7055 kj
d. 10,1365 kj

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was ne...
Questions



History, 03.05.2020 14:14


English, 03.05.2020 14:14

English, 03.05.2020 14:14


Mathematics, 03.05.2020 14:14

Mathematics, 03.05.2020 14:14

Mathematics, 03.05.2020 14:14


Mathematics, 03.05.2020 14:14

History, 03.05.2020 14:14


English, 03.05.2020 14:14

Mathematics, 03.05.2020 14:14

Mathematics, 03.05.2020 14:14

History, 03.05.2020 14:14

Mathematics, 03.05.2020 14:14

Social Studies, 03.05.2020 14:14

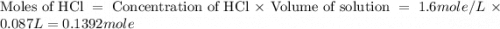
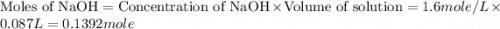

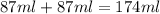
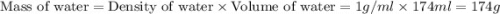
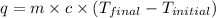
 = specific heat of water =
= specific heat of water = 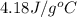
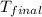 = final temperature of water = 317.4 K
= final temperature of water = 317.4 K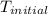 = initial temperature of metal = 298 K
= initial temperature of metal = 298 K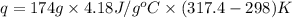
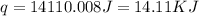
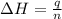
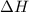 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?