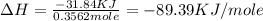Find the enthalpy of neutralization of hcl and naoh. 137 cm3 of 2.6 mol dm-3 hydrochloric acid was neutralized by 137 cm3 of 2.6 mol dm-3 naoh. the temperature rose from 298 k to 325.8 k. the specific heat capacity is the same as water, 4.18 j/k g.
a. 44.69 kj/mol
b. 6123.06 kj/mol
c. 597.46 kj/mol
d. 89.39 kj/mol

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3


Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Find the enthalpy of neutralization of hcl and naoh. 137 cm3 of 2.6 mol dm-3 hydrochloric acid was n...
Questions

Computers and Technology, 15.12.2021 18:00


Law, 15.12.2021 18:00





Mathematics, 15.12.2021 18:00

Business, 15.12.2021 18:00



Mathematics, 15.12.2021 18:00


Mathematics, 15.12.2021 18:00

Mathematics, 15.12.2021 18:00



Engineering, 15.12.2021 18:00


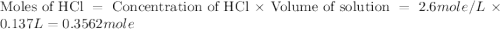
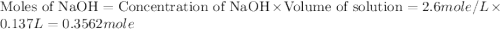

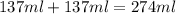
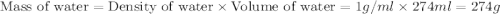
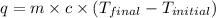
 = specific heat of water =
= specific heat of water = 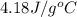
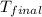 = final temperature of water = 325.8 K
= final temperature of water = 325.8 K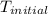 = initial temperature of metal = 298 K
= initial temperature of metal = 298 K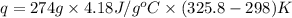
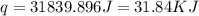
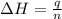
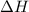 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?