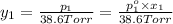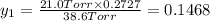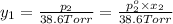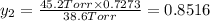Chemistry, 03.07.2019 21:20 mohamedramadan
Asolution contains two isomers, n-propyl alcohol and isopropyl alcohol, at 25°c. the total vapor pressure is 38.6 torr. what are the mole fractions of each alcohol in the liquid and in the vapor phase? the vapor pressures are 21.0 torr for n-propyl alcohol and 45.2 torr for isopropyl alcohol.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
Asolution contains two isomers, n-propyl alcohol and isopropyl alcohol, at 25°c. the total vapor pre...
Questions




Mathematics, 20.10.2020 21:01

English, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01



Mathematics, 20.10.2020 21:01

Geography, 20.10.2020 21:01



History, 20.10.2020 21:01

Biology, 20.10.2020 21:01

Chemistry, 20.10.2020 21:01



History, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01

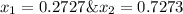 .
.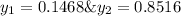 .
.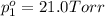
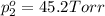
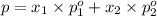 (Raoult's Law)
(Raoult's Law)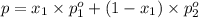
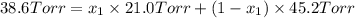
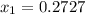
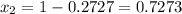
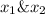 is mole fraction in liquid phase.
is mole fraction in liquid phase.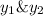
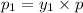 (Dalton's law of partial pressure)
(Dalton's law of partial pressure)