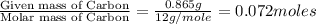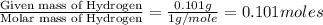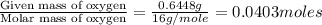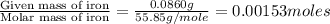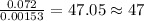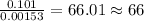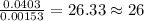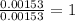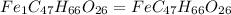Chemistry, 03.07.2019 21:20 delanieloya
When 1.6968 g of an organic iron compound containing fe, c, h, and o was burned in o2, 3.1737 g of co2 and 0.90829 g of h2o were produced. in a separate experiment to determine the mass percent of iron, 0.5446 g of the compound yielded 0.1230 g of fe2o3. what is the empirical formula of the compound?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which statement about sound is not true? a. air particles travel with sound waves. b. sound waves cannot travel through a vacuum. c. sound waves exist even if no one hears them. d. air particles vibrate along the path of a sound wave.
Answers: 1

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3
You know the right answer?
When 1.6968 g of an organic iron compound containing fe, c, h, and o was burned in o2, 3.1737 g of c...
Questions







Spanish, 17.11.2020 21:10





History, 17.11.2020 21:10

Mathematics, 17.11.2020 21:10

Mathematics, 17.11.2020 21:10


Mathematics, 17.11.2020 21:10

Mathematics, 17.11.2020 21:10



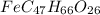
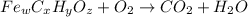
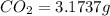
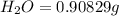
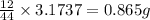 of carbon will be contained.
of carbon will be contained.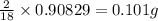 of hydrogen will be contained.
of hydrogen will be contained.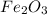 =
= 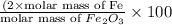
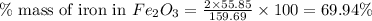
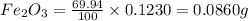 of iron.
of iron.