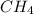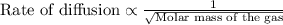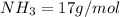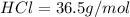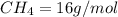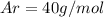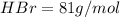Chemistry, 27.06.2019 02:10 kcceff4085
Of the following gases, will have the greatest rate of effusion at a given temperature. of the following gases, will have the greatest rate of effusion at a given temperature. nh3 hcl ch4 ar hbr

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3


You know the right answer?
Of the following gases, will have the greatest rate of effusion at a given temperature. of the foll...
Questions


Mathematics, 20.09.2020 09:01


English, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01


History, 20.09.2020 09:01




Mathematics, 20.09.2020 09:01


Advanced Placement (AP), 20.09.2020 09:01


Physics, 20.09.2020 09:01

History, 20.09.2020 09:01


Mathematics, 20.09.2020 09:01