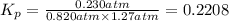Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g)+cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 480 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.820 cl2 1.27 cocl2 0.230 what is the equilibrium constant, kp, of this reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain...
Questions

Mathematics, 05.05.2020 03:05

History, 05.05.2020 03:05

Biology, 05.05.2020 03:05

English, 05.05.2020 03:05

History, 05.05.2020 03:05


Arts, 05.05.2020 03:05


Chemistry, 05.05.2020 03:05


Mathematics, 05.05.2020 03:05


Mathematics, 05.05.2020 03:05


Mathematics, 05.05.2020 03:05

Mathematics, 05.05.2020 03:05


Mathematics, 05.05.2020 03:05


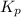 .
.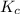
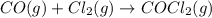
![p_{[CO]} = 0.820 atm](/tpl/images/0016/1526/aeb14.png)
![p_{[Cl_2]} = 1.27 atm](/tpl/images/0016/1526/400f0.png)
![p_{[COCl_2]} = 0.230 atm](/tpl/images/0016/1526/bbe98.png)
![K_p=\frac{p_{[COCl_2]}}{p_{[CO]}p_{[Cl_2]}}](/tpl/images/0016/1526/7cccd.png)