
Chemistry, 23.06.2019 03:40 ElegantEmerald
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh)....
Questions

Mathematics, 28.09.2021 16:20

English, 28.09.2021 16:20








Mathematics, 28.09.2021 16:20



History, 28.09.2021 16:20


Mathematics, 28.09.2021 16:20

Social Studies, 28.09.2021 16:20

Mathematics, 28.09.2021 16:20



Mathematics, 28.09.2021 16:20

 be
be  . Note that
. Note that 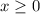 .
.
![\displaystyle \frac{[\mathrm{C_6H_5COO^{-}}]\cdot [\mathrm{H^{+}}]}{[\mathrm{C_6H_5COOH}]} = \mathrm{pK}_{a}](/tpl/images/0006/5046/265ec.png) .
.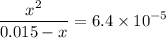 .
. :
: .
. .
. .
.![\rm [H^{+}] = 0.00306655\; mol\cdot L^{-1}](/tpl/images/0006/5046/db736.png) .
.![\displaystyle \mathrm{pH} = -\log_{10}{[\mathrm{H^{+}}]} = 2.513](/tpl/images/0006/5046/141cf.png) .
.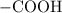 . Benzoic acid is thus a monoprotic acid. Each mole of the acid will react with only one mole of
. Benzoic acid is thus a monoprotic acid. Each mole of the acid will react with only one mole of  . The 100 mL solution initially contains
. The 100 mL solution initially contains  moles of benzoic acid. The
moles of benzoic acid. The  moles of
moles of  (from the salt
(from the salt  ) and
) and  moles of
moles of 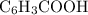 .
.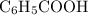 and the conjugate base of the acid
and the conjugate base of the acid  for benzoic acid.
for benzoic acid.![\begin{aligned}\mathrm{pH} &= \mathrm{pK}_{a} + \log{\frac{{[\text{Conjugate Base}]}}{[\text{Weak Acid}]}} \\ &= \mathrm{pK}_{a} + \log{\frac{{[\mathrm{C_6H_5COO^{-}}]}}{[\mathrm{C_6H_5COOH}]}}\\ &= 4.19382 + \log{\frac{0.01}{0.005}}\\ &\approx 4.495 \end{aligned}](/tpl/images/0006/5046/20b2b.png) .
.

