

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 08:00
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3
You know the right answer?
Calculate the energy in calories required to produce, from neutral he atoms,1 mole of he+ ions and h...
Questions

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Physics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

English, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Biology, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Biology, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

Mathematics, 18.09.2020 14:01

![\Delta E = R_{H}[\frac{1}{n^{2}_{i}} - \frac{1}{n^{2}_{f}}]](/tpl/images/0010/5843/a1c6b.png)
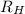 = Reydberg's constant =
= Reydberg's constant = 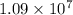 per meter
per meter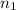 = 1 and
= 1 and 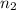 =
= 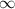
![1.09 \times 10^{7}[\frac{1}{(1)^{2}} - \frac{1}{\infty}}]](/tpl/images/0010/5843/d56c8.png) J
J ![1.09 \times 10^{7}[1 - 0}]](/tpl/images/0010/5843/bc35e.png) J
J ![1.09 \times 10^{7}]](/tpl/images/0010/5843/17892.png) J
J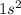 . Thus, energy required to produce
. Thus, energy required to produce 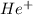 and
and 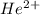 will be the same.
will be the same.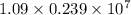 J
J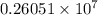 cal
cal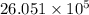 cal
cal

