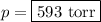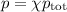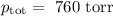Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
The mole fraction of nitrogen in the air is 0.7808. this means that 78.08% of the molecules in the a...
Questions



History, 04.08.2019 14:30

History, 04.08.2019 14:30

Chemistry, 04.08.2019 14:30

Business, 04.08.2019 14:30

Social Studies, 04.08.2019 14:30

Biology, 04.08.2019 14:30

Social Studies, 04.08.2019 14:30

History, 04.08.2019 14:30









Mathematics, 04.08.2019 14:30