Chemistry, 28.01.2020 13:51 Karinaccccc
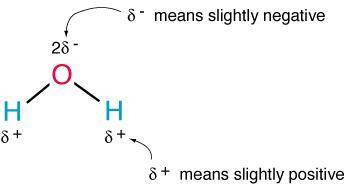
What is a result of the unequal electron sharing in a water molecule?
a. water molecules have a nonpolar bond.
b. water molecules have a weakly positive oxygen end.
c. water molecules have a weakly positive hydrogen end.
d. water molecules have two oxygen and two hydrogen atoms

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a concentration of 5.2 × 10–8 m h3o+?
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
What is a result of the unequal electron sharing in a water molecule?
a. water molecules hav...
a. water molecules hav...
Questions



Biology, 17.12.2019 18:31



English, 17.12.2019 18:31




Mathematics, 17.12.2019 18:31

Physics, 17.12.2019 18:31

Mathematics, 17.12.2019 18:31




Mathematics, 17.12.2019 18:31