
Chemistry, 31.01.2020 19:57 kimlyn58p0wyn0
Asolution is made by adding 50.0 ml of 0.200 m acetic acid (ka = 1.8 x 10–5) to 50.0 ml of 1.00 x 10–3m hcl. (a) calculate the ph of the solution.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
Asolution is made by adding 50.0 ml of 0.200 m acetic acid (ka = 1.8 x 10–5) to 50.0 ml of 1.00 x 10...
Questions

Mathematics, 29.01.2021 21:10



Mathematics, 29.01.2021 21:10

Mathematics, 29.01.2021 21:10

Chemistry, 29.01.2021 21:10






Mathematics, 29.01.2021 21:10

Business, 29.01.2021 21:10

Chemistry, 29.01.2021 21:10

English, 29.01.2021 21:10

Mathematics, 29.01.2021 21:10

Mathematics, 29.01.2021 21:10

Social Studies, 29.01.2021 21:10


Mathematics, 29.01.2021 21:10

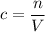 ,
, is the concentration of the solute,
is the concentration of the solute,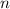 is the number of moles of the solute, and
is the number of moles of the solute, and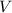 is the volume of the solution.
is the volume of the solution.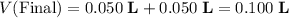 .
.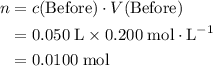 .
.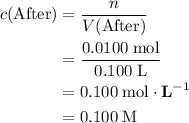 .
.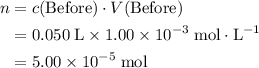 .
.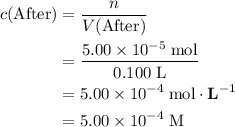 .
.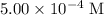 .
.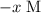 .
. 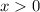 .
.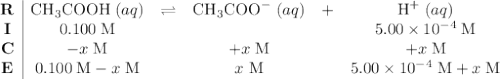 .
.![\displaystyle K_a = \frac{[\text{CH}_3\text{COO}^{-}\;(aq)]\cdot[\text{H}^{+}\;(aq)]}{[\text{CH}_3\text{COOH}\;(aq)]} = \frac{x\cdot(x + 5.00\times 10^{-4})}{0.100 - x}](/tpl/images/0490/9938/f60d8.png) .
. :
: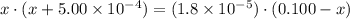
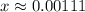 .
.![[\text{H}^{+}\;(aq)] = 5.00\times 10^{-4}\;\text{M} + x\;\text{M} = 0.00161\;\text{M}](/tpl/images/0490/9938/8318a.png) .
.![\text{pH} = -\log{[\text{H}^{+}]} = 2.79](/tpl/images/0490/9938/6dac0.png) .
.

