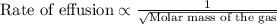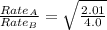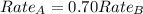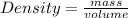Chemistry, 29.09.2019 01:30 zmoore3793
The molar mass of two equally sized samples of unknown gaseous compounds is shown in the table. molar mass comparison gas molar mass a 4.00 g/mol b 2.01 g/mol which statement describes the density and effusion of both gases at stp? gas a has a higher density and effuses faster than gas b. gas a has a higher density and effuses slower than gas b. gas a has a lower density and effuses faster than gas b. gas a has a lower density and effuses slower than gas b.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2
You know the right answer?
The molar mass of two equally sized samples of unknown gaseous compounds is shown in the table. mola...
Questions


Business, 16.01.2021 21:30

Mathematics, 16.01.2021 21:30

Health, 16.01.2021 21:30

History, 16.01.2021 21:30


Mathematics, 16.01.2021 21:30

Mathematics, 16.01.2021 21:30


Arts, 16.01.2021 21:30


History, 16.01.2021 21:30

Biology, 16.01.2021 21:30



Mathematics, 16.01.2021 21:30

Mathematics, 16.01.2021 21:30

Biology, 16.01.2021 21:30


Mathematics, 16.01.2021 21:30