Chemistry, 03.02.2020 08:57 tasnimabdallah971
Energy and specific heat
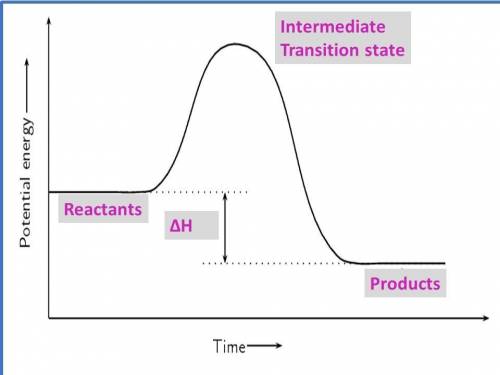
1. draw a graph of an exothermic reaction. label reactants, products and ∆h.
2. calculate the amount of energy required to raise the temperature of 3.00g of gold from 45.9 to 93.0°c.
3. 1.70g of a silvery metal requires 1000.j of energy to change its temp from 298k to 2749k. is the metal pure silver?


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

You know the right answer?
Energy and specific heat
1. draw a graph of an exothermic reaction. label reactants, prod...
1. draw a graph of an exothermic reaction. label reactants, prod...
Questions



Mathematics, 01.06.2020 22:58


Chemistry, 01.06.2020 22:58

History, 01.06.2020 22:58

Chemistry, 01.06.2020 22:58

Mathematics, 01.06.2020 22:58



World Languages, 01.06.2020 22:58





Mathematics, 01.06.2020 22:58

Mathematics, 01.06.2020 22:58