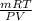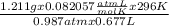Chemistry, 29.01.2020 03:59 svarner2001
Agas collected from a reaction has a mass of 1.211 g and occupies a volume of 0.677 l. the temperature in the laboratory is 296 k, and the air pressure is 0.987 atm. calculate the molar mass of the gas.
a) 23.7 g/mol
b) 44.0 g/mol
c) 58.5 g/mol
d) 82.3 g/mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1

You know the right answer?
Agas collected from a reaction has a mass of 1.211 g and occupies a volume of 0.677 l. the temperatu...
Questions





English, 04.12.2019 01:31


History, 04.12.2019 01:31





History, 04.12.2019 01:31



Mathematics, 04.12.2019 01:31

Mathematics, 04.12.2019 01:31




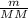 ) RT → MM =
) RT → MM =