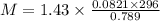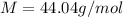Chemistry, 29.01.2020 04:43 kingken3400
The density of a gas is 1.43 g/l at a temperature of 23 ∘c and a pressure of 0.789 atm. calculate the molar mass of the gas.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2
You know the right answer?
The density of a gas is 1.43 g/l at a temperature of 23 ∘c and a pressure of 0.789 atm. calculate th...
Questions



Chemistry, 20.05.2021 21:30

Mathematics, 20.05.2021 21:30







Mathematics, 20.05.2021 21:30

Mathematics, 20.05.2021 21:30

Mathematics, 20.05.2021 21:30



Mathematics, 20.05.2021 21:30




Chemistry, 20.05.2021 21:30

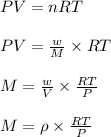
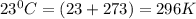
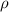 = density =1.43 g/ml
= density =1.43 g/ml