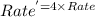Areaction in which a, b, and c react to form products is zero order in a, one-half order in b, and second order in c. by what factor does the reaction rate change if the concentration of a is doubled? 1 by what factor does the reaction rate change if the concentration of b is doubled? 1.4 by what factor does the reaction rate change if the concentration of c is doubled? 4

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1



Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Areaction in which a, b, and c react to form products is zero order in a, one-half order in b, and s...
Questions


Mathematics, 18.07.2019 14:40


Mathematics, 18.07.2019 14:40



History, 18.07.2019 14:40


History, 18.07.2019 14:40




Mathematics, 18.07.2019 14:40



Business, 18.07.2019 14:40




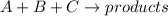
![Rate=k[A]^0[B]^\frac{1}{2}[C]^2](/tpl/images/0009/3165/73aa6.png)
![Rate^'=k[2A]^0[B]^\frac{1}{2}[C]^2](/tpl/images/0009/3165/fb0e0.png)
![Rate^'=k[2]^0[A]^0[B]^\frac{1}{2}[C]^2](/tpl/images/0009/3165/cb8ed.png)
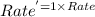
![Rate^'=k[A]^0[2B]^\frac{1}{2}[C]^2](/tpl/images/0009/3165/4f92e.png)
![Rate^'=k[A]^0[2]^\frac{1}{2}[B]^\frac{1}{2}[C]^2](/tpl/images/0009/3165/04cd0.png)
![Rate^'=[2]^\frac{1}{2}Rate](/tpl/images/0009/3165/3dc61.png)
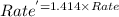
![Rate^'=k[A]^0[B]^\frac{1}{2}[2C]^2](/tpl/images/0009/3165/134f8.png)
![Rate^'=k[A]^0[B]^\frac{1}{2}[2]^2[C]^2](/tpl/images/0009/3165/77ebd.png)
![Rate^'=[2]^2Rate](/tpl/images/0009/3165/54b22.png)