
Chemistry, 25.06.2019 05:30 sanociahnoel
If 3.8 moles of zinc metal react with 6.5 moles of silver nitrate, how many moles of silver metal can be formed, and how many moles of the excess reactant will be left over when the reaction is complete? unbalanced equation: zn + agno3 → zn(no3)2 + ag be sure to show all of your work.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1
You know the right answer?
If 3.8 moles of zinc metal react with 6.5 moles of silver nitrate, how many moles of silver metal ca...
Questions

Social Studies, 02.10.2021 18:40



Mathematics, 02.10.2021 18:40

Mathematics, 02.10.2021 18:40

English, 02.10.2021 18:40

Mathematics, 02.10.2021 18:40




English, 02.10.2021 18:50




Geography, 02.10.2021 18:50

Engineering, 02.10.2021 18:50




Biology, 02.10.2021 18:50

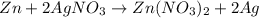
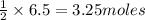 of zinc metal
of zinc metal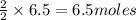 of silver metal.
of silver metal.

