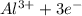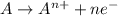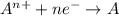Chemistry, 26.06.2019 15:00 queenkimm26
Which of the following describes an element that is oxidized in a reaction a it gains electrons during a reaction b it has a decrease in it oxidation number c al yields al3+ + 3e- d f2 + 2e- yields 2 f-

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
Which of the following describes an element that is oxidized in a reaction a it gains electrons duri...
Questions

English, 26.10.2020 21:40

Mathematics, 26.10.2020 21:40


Chemistry, 26.10.2020 21:40

Social Studies, 26.10.2020 21:40

Advanced Placement (AP), 26.10.2020 21:40

English, 26.10.2020 21:40









Mathematics, 26.10.2020 21:40

Mathematics, 26.10.2020 21:40

Mathematics, 26.10.2020 21:40

Mathematics, 26.10.2020 21:40

Advanced Placement (AP), 26.10.2020 21:40