Chemistry, 27.06.2019 08:30 daryondaniels28
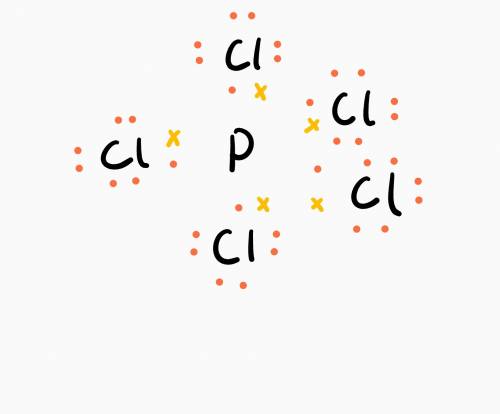
Many elements in the third row and beyond in the periodic table may form more than four bonds and thus appear to have "expanded octets." phosphorus and sulfur, for example, may form five and six covalent bonds. count up the total number of valence electrons in pcl5 and draw its lewis structure. how many valence electrons are "counted" toward the central p atom?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2
You know the right answer?
Many elements in the third row and beyond in the periodic table may form more than four bonds and th...
Questions




Social Studies, 06.10.2019 05:00

English, 06.10.2019 05:00




Health, 06.10.2019 05:00

Mathematics, 06.10.2019 05:00

Mathematics, 06.10.2019 05:00




Physics, 06.10.2019 05:00



Mathematics, 06.10.2019 05:00

Mathematics, 06.10.2019 05:00

Mathematics, 06.10.2019 05:00