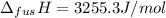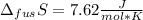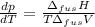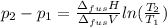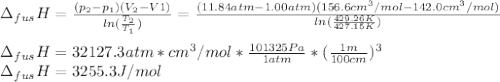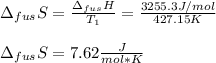The molar volume of a certain solid is 142.0 cm3 mol−1 at 1.00 atm and 427.15 k, its melting temperature. the molar volume of the liquid at this temperature and pressure is 152.6 cm3 mol−1. at 1.2 mpa the melting temperature changes to 429.26 k. calculate the enthalpy and entropy of fusion of the solid

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
The molar volume of a certain solid is 142.0 cm3 mol−1 at 1.00 atm and 427.15 k, its melting tempera...
Questions

Mathematics, 14.06.2021 05:40

Mathematics, 14.06.2021 05:40

English, 14.06.2021 05:40


Physics, 14.06.2021 05:40


Health, 14.06.2021 05:40


Mathematics, 14.06.2021 05:40


Law, 14.06.2021 05:40



Physics, 14.06.2021 05:40


Mathematics, 14.06.2021 05:40

Mathematics, 14.06.2021 05:40

Social Studies, 14.06.2021 05:40

English, 14.06.2021 05:40