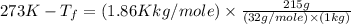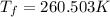Chemistry, 29.06.2019 23:30 FunnySkittle
Asolution is prepared by dissolving 215 grams of methanol, ch3oh, in 1000. grams of water. what is the freezing point of this solution? [the freezing point depression constant for water is 1.86°c/mole solute in 1000g of water]

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 23.06.2019 11:50
How many moles of an ideal gas would occupy a 25.0 liter container when the temperature is 295 k and the pressure is 0.850 atm?
Answers: 2
You know the right answer?
Asolution is prepared by dissolving 215 grams of methanol, ch3oh, in 1000. grams of water. what is t...
Questions


Mathematics, 02.11.2020 02:00

Mathematics, 02.11.2020 02:00






Health, 02.11.2020 02:00


Mathematics, 02.11.2020 02:00




Mathematics, 02.11.2020 02:00

Geography, 02.11.2020 02:00

Biology, 02.11.2020 02:00

Mathematics, 02.11.2020 02:00


Biology, 02.11.2020 02:00

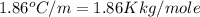
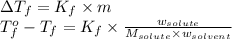
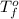 = freezing point of water =
= freezing point of water = 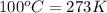
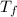 = freezing point of solution
= freezing point of solution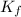 = freezing point constant
= freezing point constant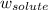 = mass of solute
= mass of solute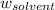 = mass of solvent
= mass of solvent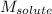 = molar mass of solute
= molar mass of solute