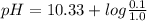Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
The pka of hco3 - (or pka2 of h2co3) is 10.33. what is the ph of a solution that has 0.1 m na2co3 an...
Questions


History, 25.02.2021 01:40



Mathematics, 25.02.2021 01:40

Mathematics, 25.02.2021 01:40


Mathematics, 25.02.2021 01:40



Mathematics, 25.02.2021 01:40

English, 25.02.2021 01:40


English, 25.02.2021 01:40

Social Studies, 25.02.2021 01:40

Chemistry, 25.02.2021 01:40


Mathematics, 25.02.2021 01:40

History, 25.02.2021 01:40

Mathematics, 25.02.2021 01:40

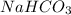 and
and 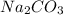 . It acts as a buffer as it is a combination of the weak acid
. It acts as a buffer as it is a combination of the weak acid 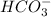 and it's conjugate base
and it's conjugate base 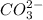 .
.![[Acid] = [HCO_{3}^{-}]=1.0 M](/tpl/images/0034/7560/4bf34.png)
![[Base] =[CO_{3}^{2-}]=0.1 M](/tpl/images/0034/7560/6fbc0.png)
![pH=pK_{a}+log\frac{[CO_{3}^{2-}]}{[HCO_{3}^{-}]}](/tpl/images/0034/7560/044bf.png)