
Chemistry, 01.07.2019 01:30 iiwolfiexuni
The hydrogen atom is not actually electronegative enough to form bonds to xenon. were the xenon-hydrogen bond to exist, what would be the structure of xeh4? double-click any atom and type xe to change the label. draw the molecule by placing atoms on the grid and connecting them with bonds. show all lone pairs of electrons.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

Chemistry, 23.06.2019 12:10
What is the correct name for hg(no3)2? mercury (i) nitrate mercury (ii) nitrate mercury nitroxide mercury dinitride
Answers: 1

Chemistry, 23.06.2019 18:00
If cos x =sin(20 + x) and 0 < x < 90, the value of x is > 3 , 35 , 350answer is 35, free 50 points
Answers: 1
You know the right answer?
The hydrogen atom is not actually electronegative enough to form bonds to xenon. were the xenon-hydr...
Questions








Business, 25.07.2019 21:40









Mathematics, 25.07.2019 21:40

History, 25.07.2019 21:40

Mathematics, 25.07.2019 21:40

Business, 25.07.2019 21:40

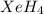 will be square-planar.
will be square-planar.

