
After the completion of a gas forming reaction, the column of water remaining in the collection flask was measured to me 55mm high, the vapor pressure of water is 0.0313atm at 25oc, and the atmospheric pressure that day was measured as 0.950 atm, what is the partial pressure of the gas produced by the reaction? 1mmhg=13.595mm h2o and 1atm=760mmhg

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
After the completion of a gas forming reaction, the column of water remaining in the collection flas...
Questions

Chemistry, 14.11.2020 06:00






Biology, 14.11.2020 06:00



Mathematics, 14.11.2020 06:00

Spanish, 14.11.2020 06:00


Mathematics, 14.11.2020 06:00



Mathematics, 14.11.2020 06:00

Business, 14.11.2020 06:00


Spanish, 14.11.2020 06:00

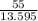 = 4.045 mm of Hg [As 1 mm of Hg is equivalent to 13.595 mm of water]
= 4.045 mm of Hg [As 1 mm of Hg is equivalent to 13.595 mm of water]


