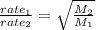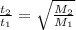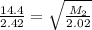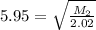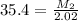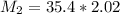Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2

Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
Asample of an unknown gas effuses in 14.4 min. an equal volume of h2 in the same apparatus under the...
Questions



Mathematics, 15.01.2021 08:10

Mathematics, 15.01.2021 08:10


History, 15.01.2021 08:10


Biology, 15.01.2021 08:10

Mathematics, 15.01.2021 08:10

Chemistry, 15.01.2021 08:10


Social Studies, 15.01.2021 08:10

Mathematics, 15.01.2021 08:10

Mathematics, 15.01.2021 08:10

Mathematics, 15.01.2021 08:10


Chemistry, 15.01.2021 08:10


Mathematics, 15.01.2021 08:10

Social Studies, 15.01.2021 08:10