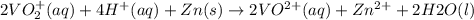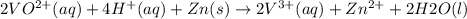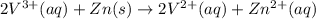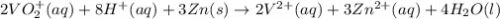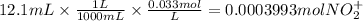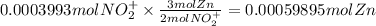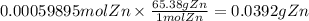Chemistry, 07.07.2019 19:30 tladitidimatso1783
The balanced redox reactions for the sequential reduction of vanadium are given below. reduction from +5 to +4: 2 vo2+(aq) + 4 h +(aq) + zn(s) → 2 vo2+(aq) + zn2+(aq) + 2 h2o(l) reduction from +4 to +3: 2 vo2+(aq) + zn(s) + 4 h +(aq) → 2 v3+(aq) + zn2+(aq) + 2 h2o(l) reduction from +3 to +2: 2 v3+(aq) + zn(s) → 2 v2+(aq) + zn2+(aq) if you had 12.1 ml of a 0.0033 m solution of vo2+(aq), how many grams of zn metal would be required to completely reduce the vanadium?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
The balanced redox reactions for the sequential reduction of vanadium are given below. reduction fro...
Questions

History, 15.10.2019 10:30





Mathematics, 15.10.2019 10:30

Mathematics, 15.10.2019 10:30

English, 15.10.2019 10:30



History, 15.10.2019 10:30




French, 15.10.2019 10:30


Mathematics, 15.10.2019 10:30

Chemistry, 15.10.2019 10:30


Mathematics, 15.10.2019 10:30