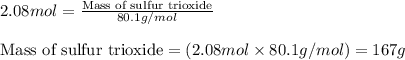Chemistry, 09.07.2019 12:30 doggosbepis
The reaction of sulfur with oxygen is shown below. 2s(s) + 3o2(g) → 2so3(g) calculate the mass of sulfur trioxide (in g) produced when 100.0 g of each reactant is present.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
You know the right answer?
The reaction of sulfur with oxygen is shown below. 2s(s) + 3o2(g) → 2so3(g) calculate the mass of su...
Questions

Mathematics, 08.11.2020 23:40

Social Studies, 08.11.2020 23:40


Engineering, 08.11.2020 23:40

Mathematics, 08.11.2020 23:40


English, 08.11.2020 23:40

English, 08.11.2020 23:40


English, 08.11.2020 23:40


History, 08.11.2020 23:40



Arts, 08.11.2020 23:40


English, 08.11.2020 23:40



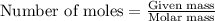 .....(1)
.....(1)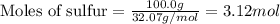
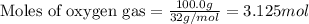
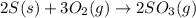
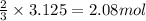 of sulfur metal
of sulfur metal