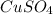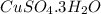Chemistry, 10.07.2019 21:00 saltedcaramel60
In another experiment, you have 4.5000 g of a copper(ii) sulfate hydrate with an unknown number of attached water molecules. after heating the hydrate, you have 3.3608 g of the anhydrous compound (copper(ii) sulfate with no waters) left. using these data, calculate the number of water molecules that is present in the formula of this hydrate (obviously before heating).

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1
You know the right answer?
In another experiment, you have 4.5000 g of a copper(ii) sulfate hydrate with an unknown number of a...
Questions

Chemistry, 17.11.2020 19:40

Mathematics, 17.11.2020 19:40

Biology, 17.11.2020 19:40





Chemistry, 17.11.2020 19:40

Mathematics, 17.11.2020 19:40



Mathematics, 17.11.2020 19:40




Mathematics, 17.11.2020 19:40



Physics, 17.11.2020 19:40

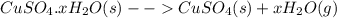
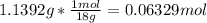
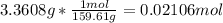
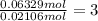
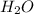 per one mol
per one mol