
Chemists often use molarity m, in moles/liter, to measure the concentration of solutions. molarity is a common unit of concentration because the volume of a liquid is very easy to measure. however, the drawback of using molarity is that volume is a temperature-dependent quantity. as temperature changes, density changes, which affects volume. volume markings for most laboratory glassware are calibrated for room temperature, about 20∘c. fortunately, there are several other ways of expressing concentration that do not involve volume and are therefore temperature independent. a 2.800×10−2 m solution of nacl in water is at 20.0∘c. the sample was created by dissolving a sample of nacl in water and then bringing the volume up to 1.000 l. it was determined that the volume of water needed to do this was 999.2 ml . the density of water at 20.0∘c is 0.9982 g/ml.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Litmus paper is made from water-soluble dyes which are extracted from lichens. this paper is used as an acid-base indicator. which of these common household substances would turn blue litmus paper red? a) bleach b) lye c) soap d) vinegar
Answers: 3

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1
You know the right answer?
Chemists often use molarity m, in moles/liter, to measure the concentration of solutions. molarity i...
Questions

English, 12.01.2021 17:50

Mathematics, 12.01.2021 17:50

Mathematics, 12.01.2021 17:50

Mathematics, 12.01.2021 17:50



Mathematics, 12.01.2021 17:50


English, 12.01.2021 17:50



Mathematics, 12.01.2021 17:50

Mathematics, 12.01.2021 17:50



Mathematics, 12.01.2021 17:50

English, 12.01.2021 17:50

Social Studies, 12.01.2021 17:50

Mathematics, 12.01.2021 17:50

German, 12.01.2021 17:50

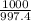 x 2.800×10⁻² moles of NaCl.
x 2.800×10⁻² moles of NaCl.


