Chemistry, 12.07.2019 12:30 markmlg122
An unknown compound with a molar mass of 155.06 g/mol consists of 46.47% c, 7.80% h, and 45.72% cl. find the molecular formula for the compound. c6h12cl2 chcl c9h18cl3 c6h12cl

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
An unknown compound with a molar mass of 155.06 g/mol consists of 46.47% c, 7.80% h, and 45.72% cl....
Questions




Mathematics, 15.07.2021 20:50



English, 15.07.2021 20:50




Mathematics, 15.07.2021 20:50

Mathematics, 15.07.2021 20:50

Mathematics, 15.07.2021 20:50


Mathematics, 15.07.2021 20:50

English, 15.07.2021 20:50

Mathematics, 15.07.2021 20:50



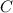 :
: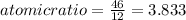
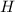 :
: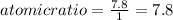
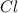 :
: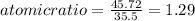
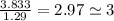
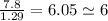
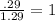
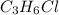 .
.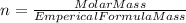
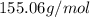

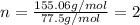
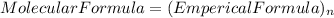 so, the molecular formula is:
so, the molecular formula is: