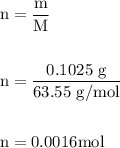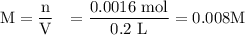Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
A0.1025-g sample of copper metal is dissolved in 35 ml of concentrated hno3 to form cu2 ions and the...
Questions



Mathematics, 26.02.2021 01:00

History, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00

Health, 26.02.2021 01:00





Mathematics, 26.02.2021 01:00


Mathematics, 26.02.2021 01:00



French, 26.02.2021 01:00

History, 26.02.2021 01:00

 Cu(NO₃)₂ + NO₂ + H₂O
Cu(NO₃)₂ + NO₂ + H₂O