
Chemistry, 21.09.2019 18:20 alondrachon
Citric acid is composed of only carbon, hydrogen, and oxygen. when a 0.5000 g sample of citric acid was burned, it produced 0.6871 g of co2 and 0.1874 g of h2o. the molar mass of the compound is 192 g/mol. what are the empirical and molecular formulas of citric acid

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1


Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
Citric acid is composed of only carbon, hydrogen, and oxygen. when a 0.5000 g sample of citric acid...
Questions







Mathematics, 19.11.2020 05:10

History, 19.11.2020 05:10


English, 19.11.2020 05:10

Mathematics, 19.11.2020 05:10

Geography, 19.11.2020 05:10


Mathematics, 19.11.2020 05:10


Mathematics, 19.11.2020 05:10

Mathematics, 19.11.2020 05:10

History, 19.11.2020 05:10

History, 19.11.2020 05:10

Mathematics, 19.11.2020 05:10

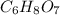 is empirical formula as well as molecular formula.
is empirical formula as well as molecular formula.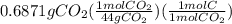
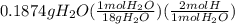
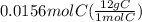
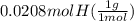
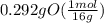
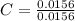 = 1
= 1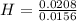 = 1.33
= 1.33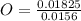 = 1.17
= 1.17


