

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
If the ka of a monoprotic weak acid is 5.4 × 10-6, what is the ph of a 0.14 m solution of this acid?...
Questions

Mathematics, 12.12.2020 16:00






Mathematics, 12.12.2020 16:00

Computers and Technology, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00


English, 12.12.2020 16:00

English, 12.12.2020 16:00

History, 12.12.2020 16:00


Mathematics, 12.12.2020 16:00


Mathematics, 12.12.2020 16:00



Mathematics, 12.12.2020 16:00

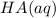 resembles the acid; as a weak acid (a small value of
resembles the acid; as a weak acid (a small value of 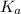 )
) 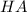 would partially dissociate to produce protons
would partially dissociate to produce protons 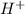 and
and 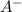 , its conjugate base. Let the final proton concentration (i.e.,
, its conjugate base. Let the final proton concentration (i.e., ![[H^{+}]](/tpl/images/0083/5038/cab36.png) ) be
) be  . (Apparently
. (Apparently 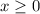 ) Construct the following RICE table:
) Construct the following RICE table: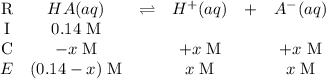
![\left\begin{array}{ccc}K_{a}&=&[H^{+}] \cdot [A^{-}] / [HA]\\&=&x^{2} /(0.14 - x)\end{array}\right](/tpl/images/0083/5038/ec898.png)
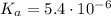
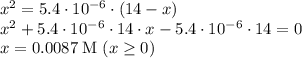
![\text{pH} = -\text{ln(}[H^{+}]\text{)} / \text{ln(}10\text{)} = 2.1](/tpl/images/0083/5038/74ad5.png)


