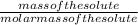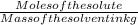Chemistry, 14.07.2019 04:00 alexcute965
Asolution is made by dissolving 21.5 grams of glucose (c6h12o6) in 255 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2


Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
Asolution is made by dissolving 21.5 grams of glucose (c6h12o6) in 255 grams of water. what is the f...
Questions

Mathematics, 20.01.2021 08:10

Mathematics, 20.01.2021 08:10





Mathematics, 20.01.2021 08:10




English, 20.01.2021 08:10

Mathematics, 20.01.2021 08:10

Mathematics, 20.01.2021 08:10


Biology, 20.01.2021 08:10

Mathematics, 20.01.2021 08:10

Mathematics, 20.01.2021 08:10

Mathematics, 20.01.2021 08:10


Physics, 20.01.2021 08:10