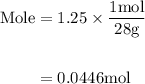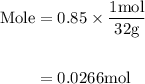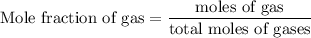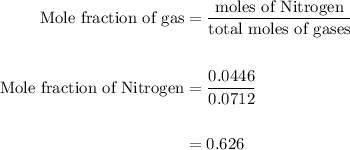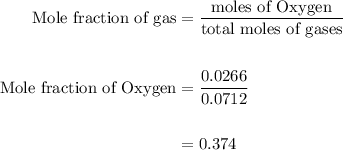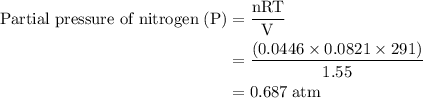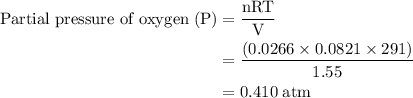Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
Agas mixture contains 1.25 g n2 and 0.85 g o2 in a 1.55 l c ontainer at 18 °c. calculate the mole fr...
Questions







Social Studies, 26.03.2020 02:36




Mathematics, 26.03.2020 02:36


Social Studies, 26.03.2020 02:37

Mathematics, 26.03.2020 02:37


Computers and Technology, 26.03.2020 02:37

Biology, 26.03.2020 02:37

Mathematics, 26.03.2020 02:37

Computers and Technology, 26.03.2020 02:37