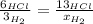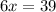Chemistry, 14.07.2019 22:30 maryalice2002
When 6.0 mol al react with 13 mol hcl, what is the limiting reactant, and how many moles of h2 can be formed? 2 al + 6hcl → 2 alcl3 + 3 h2 select one: a. al is the limiting reactant, 9.0 mol h2 can be formed b. hcl is the limiting reactant, 6.5 mol h2 can be formed c. al is the limiting reactant, 6.0 mol h2 can be formed d. hcl is the limiting reactant, 4.3 mol h2 can be formed

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1


Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1
You know the right answer?
When 6.0 mol al react with 13 mol hcl, what is the limiting reactant, and how many moles of h2 can b...
Questions


English, 18.04.2020 17:20

Mathematics, 18.04.2020 17:20


Chemistry, 18.04.2020 17:20



Mathematics, 18.04.2020 17:20

Computers and Technology, 18.04.2020 17:20

Mathematics, 18.04.2020 17:20




Computers and Technology, 18.04.2020 17:21

Mathematics, 18.04.2020 17:21

Mathematics, 18.04.2020 17:21

Mathematics, 18.04.2020 17:21

Mathematics, 18.04.2020 17:21

Mathematics, 18.04.2020 17:21


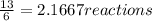
 that are produced with 13 moles of HCl.
that are produced with 13 moles of HCl.