
Chemistry, 15.07.2019 20:30 Badbpyz7550
Calculate the heat released when 5.00 l of cl2(g) with a density of 1.88 g/l reacts with an excess of sodium metal at 25°c and 1 atm to form sodium chloride.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Calculate the heat released when 5.00 l of cl2(g) with a density of 1.88 g/l reacts with an excess o...
Questions

Mathematics, 05.02.2021 01:00




Mathematics, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

Physics, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

English, 05.02.2021 01:00

English, 05.02.2021 01:00

Health, 05.02.2021 01:00

Health, 05.02.2021 01:00


Mathematics, 05.02.2021 01:00

English, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

Biology, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

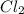 which has density of 1.88 g
which has density of 1.88 g

