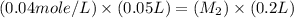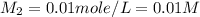Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes or no?
Answers: 1
You know the right answer?
If water is added to 50 ml of a 0.04 m solution so that it fills a 200 ml beaker, what is the final...
Questions



History, 26.08.2019 20:10





History, 26.08.2019 20:10


History, 26.08.2019 20:10



History, 26.08.2019 20:10





Mathematics, 26.08.2019 20:10


History, 26.08.2019 20:10

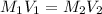
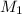 = initial molarity (concentration) of solution = 0.04 M = 0.04 mole/L
= initial molarity (concentration) of solution = 0.04 M = 0.04 mole/L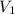 = initial volume of solution = 50 ml = 0.05 L
= initial volume of solution = 50 ml = 0.05 L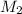 = final molarity (concentration) of solution = ?
= final molarity (concentration) of solution = ?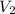 = final volume of solution = 200 ml = 0.2 L
= final volume of solution = 200 ml = 0.2 L